Answer:
Step-by-step explanation:
We are given that an equation
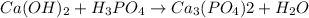
We have to find the proper way to balance the equation
Calculate number of atoms each type of element on left hand side and on right side
On left hand side
Number of atoms of Ca=1
Number of oxygen atoms=6
Number of phosphorous atom=1
Number of hydrogen atoms=5
On right hand side
Number of Ca atoms=3
Number of oxygen atoms=9
Number of phosphorous atom=2
Number of hydrogen atoms=2
We substitute 3 as stoichiometric coefficient of
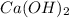 and 2 as stoichiometric coefficient of
and 2 as stoichiometric coefficient of
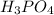
Then, the equation
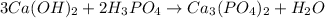
Now,Number of oxygen on left side =14
Number of hydrogen atom on left side =12
Therefore, we substituting 6 as stoichiometric coefficient of water
Then ,we get
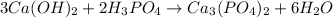
Now, we have on left side
Total number of oxygen atom=14
Total number of hydrogen atom=12
Total number of phosphorous atom=2
Total number of Ca atom=3
On right side
Total number of oxygen atom=14
Number of hydrogen atom=12
Number of phosphorous atom=2
Number of Ca atom=3
Hence, the equation is balance.