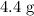 .
.
Step-by-step explanation
Refer to a modern periodic table for relative atomic mass:
Formula of copper (II) chloride: CuCl₂.
Formula mass of copper (II) chloride:
63.546 + 2 × 35.45 =
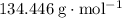 .
.
How many
 of formula units in 8.3 grams of CuCl₂?
of formula units in 8.3 grams of CuCl₂?
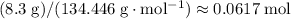
Note the subscript "2" in the formula CuCl₂. There are
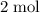 of chloride ions in every
of chloride ions in every
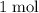 formula unit of CuCl₂.
formula unit of CuCl₂.
There would be
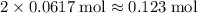 of
of
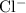 ions in
ions in
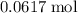 formula units of CuCl₂.
formula units of CuCl₂.
The mass of
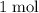 of chloride ion is 35.45 grams.
of chloride ion is 35.45 grams.
The mass of
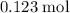 of
of
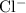 ions will be
ions will be
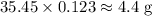 .
.
Round the final value to two sig. fig. since the mass in the question is also 2 sig. fig.