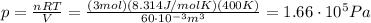Answer:
Step-by-step explanation:
The ideal gas equation states that:
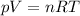
where
p is the gas pressure
V is the gas volume
n is the number of moles of the gas
R is the gas constant
T is the absolute temperature of the gas
For the gas in this problem, we have:
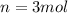 (number of moles)
(number of moles)
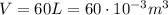 (gas volume)
(gas volume)
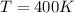
So we can solve the formula for p, the pressure of the gas: