Answer:
A half-life is the time required for one half of the nuclei in a radio- active isotope to decay.
Step-by-step explanation:
A radio-active isotope is an isotope which undergoes radioactive decay.
Radioactive decay is a spontaneous process in which the nucleus of an atom changes its state (turning into a different nucleus, or de-exciting), emitting radiation, which can be of three different types: alpha, beta or gamma.
The half-life of a radio-active isotope is the time required for half of the nuclei of the initial sample to decay.
The law of radio-active decay can be expressed as follows:
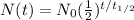
where
N(t) is the number of undecayed nuclei left at time t
N0 is the initial number of nuclei
t is the time
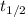 is the half-life
is the half-life
We see that when
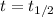 (that means, when 1 half-life has passed), the number of undecayed nuclei left is
(that means, when 1 half-life has passed), the number of undecayed nuclei left is
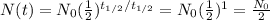
So, half of the initial nuclei.