Hello! :)
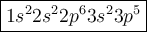
Chlorine is a halogen located in group 7A and period 3 of the periodic table.
We can write the electron configuration of this element. Since it is in period 3, the highest configuration level will be at 3.
Chlorine is also located in the p block (nonmetal) section of the table, so the final part of the written configuration will involve "3p".
Recall that:
S block: up to 2 electrons
P block: up to 6 electrons
Chlorine has 17 electrons
Fill in the order of s block to p block. The first level only goes up to an s block. The configuration should sum up to 17 total electrons in total.
We can write the configuration as: