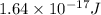Answer: The kinetic energy of the electron is
Step-by-step explanation:
To calculate the kinetic energy of the electron, we use the equation:
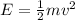
where,
m = mass of the electron =
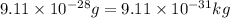 (Conversion factor: 1 kg = 1000 g)
(Conversion factor: 1 kg = 1000 g)
v = speed of the electron =
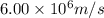
Putting values in above equation, we get:
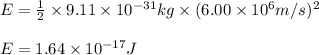
Hence, the kinetic energy of the electron is