Answer: 0.129 moles of gas were added to the bottle
Step-by-step explanation:
According to the ideal gas equation:'

P = Pressure of the gas
V= Volume of the gas
T= Temperature of the gas
R= Gas constant
n= moles of gas
As Volume , gas constant and temperature are constant
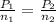
where,
 = initial pressure of gas =730 mm Hg =0.960 atm (760 mmHg = 1 atm)
= initial pressure of gas =730 mm Hg =0.960 atm (760 mmHg = 1 atm)
 = final pressure of gas = 1.15 atm
= final pressure of gas = 1.15 atm
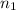 = initial number of moles = 0.650
= initial number of moles = 0.650
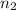 = final number of moles = ?
= final number of moles = ?
Now put all the given values in the above equation, we get the final moles of gas.
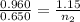
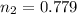
Therefore, the number of moles of gas will be 0.779
Thus moles of gas were added to the bottle are (0.779-0.650) = 0.129