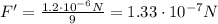1. When the distance is doubled:
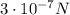
The electrostatic force between two charges is given by:
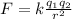
where
k is the Coulomb's constant
q1 and q2 are the two charges
r is the distance between the two charges
The initial force between the two charges is
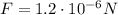 . In this part of the problem, the distance between the two charges is doubled, so we can write
. In this part of the problem, the distance between the two charges is doubled, so we can write
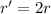
And substituting into the formula, we find the new force:
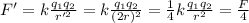
So, the force is reduced to 1/4 of its original value. Therefore, it is
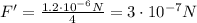
2. When the distance is halved:
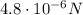
The initial force between the two charges is
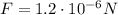 . In this part of the problem, the distance between the two charges is halved, so we can write
. In this part of the problem, the distance between the two charges is halved, so we can write
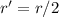
And substituting into the formula, we find the new force:
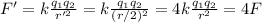
So, the force is quadrupled. Therefore, it is
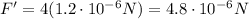
3. When the distance is tripled:
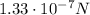
The initial force between the two charges is
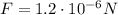 . In this part of the problem, the distance between the two charges is tripled, so we can write
. In this part of the problem, the distance between the two charges is tripled, so we can write
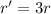
And substituting into the formula, we find the new force:
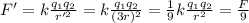
So, the force is reduced to 1/9 of its original value. Therefore, it is