Answer:
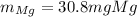
Step-by-step explanation:
Hello,
Based on the given chemical reaction, as 31.2 mL of hydrogen are yielded, one computes its moles via the ideal gas equation under the stated conditions as shown below:
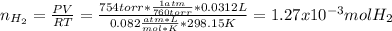
Now, since the relationship between hydrogen and magnesium is 1 to 1, one computes its milligrams by following the shown below proportional factor development:
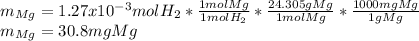
Best regards.