A. How many Joules of energy are required to raise 3.50kg of water from 38.5 to 75.0C ? (specific heat of water is 4.184J/g C)(1000g=1kg)
Answer:
q = 534,506 Joules
q = 534.506 KiloJoules
534.506 KiloJoules or 534,506 Joules energy required to raise 3.50kg of water from 38.5 to 75.0C
What is sp.heat ?
Sp. Heat is the heat required to increase the temperature of the 1 mass of a given substance by a
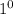 C temperature.
C temperature.
The formula of specific heat Cp =
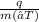
Where,
q = energy of substance (Joules / KiloJoules),
Cp = Specific heat capacity of the substance (J/Kg.C),
m = mass of the substance
∆T = Change in temp.
Step-by-step explanation:
Given data from que: -
Mss of the water (m) = 3.50 Kg = 3.50 × 1000 gm = 3500 gm
specific heat capacity of water (Cp) = 4.184J/g C
Change in temp (∆T) = 75.0 - 38.5 = 36.5
now, put all above given data in formula
we get
Cp =
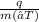
q = Cp ×m×∆T
q = 4.184×3500×36.5
q = 534,506 Joules
q = 534.506 KJ