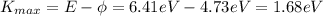(a) 263 nm
First of all, let's convert the work function for silver from eV to Joules:
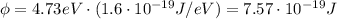
The energy of the incoming photon is given by:

where h is the Planck constant, c is the speed of light,
 is the photon's wavelength.
is the photon's wavelength.
The cutoff wavelength is the minimum wavelength for which the photon has enough energy to extract the photoelectron from the material: that means, the wavelength at which the energy of the photon is at least equal to the work function of the material,
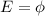
Substituting and solving for the wavelength,
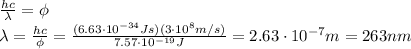
(b)
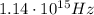
The lowest frequency of light incident on silver that releases photoelectrons from its surface is the frequency corresponding to the wavelength we found at point (a); using the relationship between frequency and wavelength:
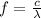
And substituting numbers, we find
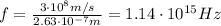
(c) 1.68 eV
The equation for the photoelectric effect is:
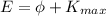
where
E is the energy of the incoming photon
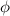 is the work function
is the work function
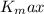 is the maximum kinetic energy of the photoelectrons
is the maximum kinetic energy of the photoelectrons
Since
E = 6.41 eV
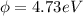
The maximum kinetic energy of the photoelectrons is