12: Helium
Step-by-step explanation:
In the picture:
- Red particles correspond to protons (because they are in the nucleus and they are positively charged)
- Green particles correspond to neutrons (because they are in the nucleus and they are uncharged)
- Yellow particles correspond to electrons (because they orbit the nucleus and they are negatively charged)
This atom as 2 protons and 2 electrons. This means it corresponds to the 2nd element in the periodic table (atomic number = 2), so it corresponds to helium.
13: Electrons should be arranged in 3 separate orbitals: 2 electrons in an orbital closer to the nucleus, another 2 in a second orbital, and the remaining 3 in the last orbital
Step-by-step explanation:
The electron configuration for nitrogen is:
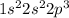
which means:
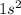 --> the closest orbital to the nucleus, energy level = 1, orbital s, containing 2 electrons
--> the closest orbital to the nucleus, energy level = 1, orbital s, containing 2 electrons
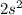 --> second closest orbital to the nucleus, energy level = 2, orbital s, containing 2 electrons
--> second closest orbital to the nucleus, energy level = 2, orbital s, containing 2 electrons
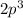 --> third closest orbital to the nucleus, energy level = 3, orbital p, containing 3 electrons
--> third closest orbital to the nucleus, energy level = 3, orbital p, containing 3 electrons