Answer:
Mass in grams of formaldehyde = 196.51 g
Step-by-step explanation:
No. of molecules of formaldehyde =

Moles is related with number of molecules as:
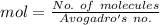
Avogadro's no. =
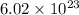
moles of formaldehyde
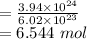
Molar mass of formaldehyde = 30.03 g/mol
Molar mass is related with mass as:
Mass = Moles × Molar mass
= 6.544 × 30.03
= 196.51 g