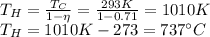(b) 71%
The thermal efficiency of a Carnot heat engine is given by:
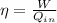
where
W is the useful work done by the engine
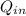 is the heat in input to the machine
is the heat in input to the machine
In this problem, we have:
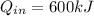 is the heat absorbed
is the heat absorbed
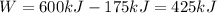 is the work done (175 kJ is the heat released to the sink, therefore the work done is equal to the difference between the heat in input and the heat released)
is the work done (175 kJ is the heat released to the sink, therefore the work done is equal to the difference between the heat in input and the heat released)
So, the efficiency is
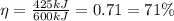
(a)
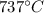
The efficiency of an engine can also be rewritten as
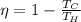
where
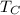 is the absolute temperature of the cold sink
is the absolute temperature of the cold sink
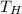 is the temperature of the source
is the temperature of the source
In this problem, the temperature of the sink is
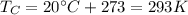
So we can re-arrange the equation to find the temperature of the source: