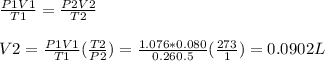Answer:
Volume of the gas is 90.2 ml
Step-by-step explanation:
Given:
Initial volume, V1 = 80.0 ml = 0.080 L
Initial pressure, P1 = 109 kPa = 1.076 atm ( 1 kPa = 0.00987 atm)
Initial temperature, T1 = -12.5 C = -12.5 + 273 K = 260.5 K
Under STP conditions i.e. standard temperature and pressure we have:
Pressure, P2 = 1 atm
Temperature, T2 = 273 K
Formula:
Based on the ideal gas relation we have: