That will make a gold-202 nucleus.
Step-by-step explanation
Refer to a periodic table. The atomic number of mercury Hg is 80.
Step One: Bombard the
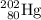 with a neutron
with a neutron
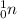 . The neutron will add 1 to the mass number 202 of
. The neutron will add 1 to the mass number 202 of
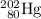 . However, the atomic number will stay the same.
. However, the atomic number will stay the same.
- New mass number: 202 + 1 = 203.
- Atomic number is still 80.
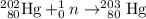 .
.
Double check the equation:
- Sum of mass number on the left-hand side = 202 + 1 = 203 = Sum of mass number on the right-hand side.
- Sum of atomic number on the left-hand side = 80 = Sum of atomic number on the right-hand side.
Step Two: The
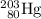 nucleus loses a proton
nucleus loses a proton
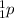 . Both the mass number 203 and the atomic number will decrease by 1.
. Both the mass number 203 and the atomic number will decrease by 1.
- New mass number: 203 - 1 = 202.
- New atomic number: 80 - 1 = 79.
Refer to a periodic table. What's the element with atomic number 79? Gold Au.
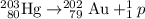 .
.
Double check the equation:
- Sum of mass number on the left-hand side = 203 = 202 + 1 = Sum of mass number on the right-hand side.
- Sum of atomic number on the left-hand side = 80 = 79 + 1 = Sum of atomic number on the right-hand side.
A gold-202 nucleus is formed.