Answer: The balanced chemical equation is written below.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
The reactivity of metal is determined by a series known as reactivity series. The metals lying above in the series are more reactive than the metals which lie below in the series.

The chemical equation for the reaction of silver sulfide with aluminium follows:
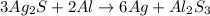
By Stoichiometry of the reaction:
3 moles of silver sulfide reacts with 2 moles of aluminium metal to produce 6 moles of silver metal and 1 mole of aluminium sulfide
Hence, the balanced chemical equation is written above.