Answer : The correct option is, (B) the value of q is positive.
Explanation :
Photosynthesis : It is a chemical process or a reaction which takes place in the green plants or the living organisms. During this process, the carbon dioxide reacts with the water in the presence of sunlight to gives glucose and oxygen as a product.
The balanced chemical reaction will be,
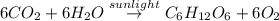
As we know that the photosynthesis reaction can not takes place without sunlight. So, the photosynthesis reaction is an endothermic reaction in which the energy or heat are given to the system. That means the value of 'q' will be positive. If heat is released by the system then the value of 'q' is negative.
Hence, the correct option is, (B) the value of q is positive.