Answer:
The final temperature is
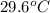 .
.
Step-by-step explanation:
When the two masses come in contact, one releases heat and the other absorbs it. The former can be modelled with the equation
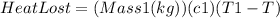 , and the latter by
, and the latter by
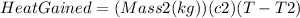
m1=0.15 kg
m2=0.06 kg
T1 = 70 degrees Celsius = 343 K
T2 = 20 degrees Celsius = 293 K
T= Final temperature
c1 = Specific heat capacity of copper
c2 = Specific heat capacity of water
Because there is no heat lost into the surroundings, the heat removed from one substance is the same as the heat gained in the other. Therefore:
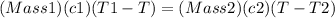
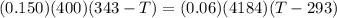
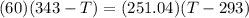
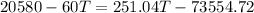
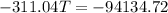
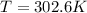
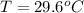
Hope this helps! (My apologies if the answer is wrong, it has been a while since I've done this)