Oxidation state on
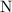 as in the nitrate ion
as in the nitrate ion
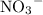 : +5.
: +5.
Step-by-step explanation
The sum of oxidation states on all atoms in an ion should be the same as the charge of the ion.
The oxidation state of nitrogen N tends to vary. However, the oxidation state of oxygen O is -2 in most cases, with the following exceptions:
- Oxidation state of O in
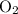 : 0.
: 0. - Oxidation state of O in
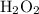 and other peroxides: -1.
and other peroxides: -1. - The oxidation state of O will be positive when it is bonded to fluorine F.
To find the oxidation state on N, consider the atoms in a nitrate ion:
- There are three O atoms in each nitrate ion, as seen in the subscript "3".
- There's only one N atom in each nitrate ion.
The oxidation state of each O atom is expected to be -2. There are three O atoms in each nitrate ion. The oxidation state of the three atoms will add up to
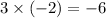 .
.
The oxidation state of the N atom needs to be found. Charge on the nitrate ion is -1. Thus
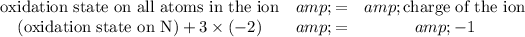
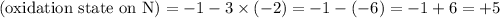 .
.
Therefore, the oxidation state of the nitrogen N atom in the nitrate ion
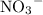 is +5.
is +5.