Step-by-step explanation:
When pH of the solution is 11.
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
![11=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/eevbgekrijm1n1z7jbqxfrtu9b7owxa5kx.png)
![[H^+]=1* 10^(-11) M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xpp1cq53zv6v7o69rphcltm8w5ii0p6dew.png) ..(1)
..(1)
At pH = 11, the concentration of
 ions is
ions is
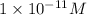 .
.
When the pH of the solution is 6.
![pH=-\log[H^+]'](https://img.qammunity.org/2020/formulas/chemistry/middle-school/mve4qvcmnwhog57n4f179qjs9yk1k2m440.png)
![6=-\log[H^+]'](https://img.qammunity.org/2020/formulas/chemistry/middle-school/9ydyyczcceanvm3bt7gqi0ijnte2vlviyi.png)
![[H^+]'=1* 10^(-6) M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/93k1r7ykhuits2pfkd8fwho0gs2fyy34wt.png) ..(2)
..(2)
At pH = 6, the concentration of
 ions is
ions is
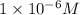 .
.
On dividing (1) by (2).
![([H^+])/([H^+]')=(1* 10^(-11) M)/(1* 10^(-6))=1* 10^(-5)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5b443vcjgfy83t40a1ddwzwmwgzgew5k4f.png)
The ratio of hydrogen ions in solution of pH equal to 11 to the solution of pH equal to 6 is
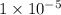 .
.
Difference between the
 ions at both pH:
ions at both pH:
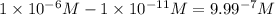
This means that Hydrogen ions in a solution at pH = 7 has
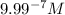 ions fewer than in a solution at a pH = 6
ions fewer than in a solution at a pH = 6