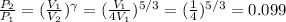Answer:
e) 0.099
Step-by-step explanation:
For an adiabatic expansion:
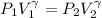
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
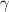 is the adiabatic index (which is
is the adiabatic index (which is
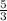 for an ideal monoatomic gas
for an ideal monoatomic gas
In this problem, we have
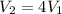 since the volume increases by a factor 4
since the volume increases by a factor 4
We can re-write the equation to find by what factor the pressure changes: