Answer: The element is Magnesium.
Step-by-step explanation:
The energy levels determine the period of the element. Thus an element with 3 energy levels will be an element of third period.
As the valence electrons are 2 , the group number of the element is 2.
Thus the element will have an electroni configuration of
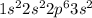
The atomic number will be equal to the number of electrons and thus atomic number will be (2+2+6+2) = 12. The element will be magnesium with atomic symbol of Mg.