Answer:
![Kw = [H+][OH-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/nn74senmqpnf96tdf80kgwuh0i2fc22fl3.png)
Step-by-step explanation:
For a given solution H+ refers to the hydrogen ion concentration and is indicative of the acidity of the solution. The more the [H+] concentration, higher is the acidity. Similarly, OH- refers to the hydroxide ion concentration and reflects the basicity of the solution. A solution with a higher OH- concentration is said to be basic.
The two are related by considering the dissociation of water:
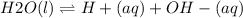
The dissociation constant for water, Kw is:
![Kw = [H+][OH-]\\\\where\ Kw = 10^(-14) \\\\10^(-14) = [H+][OH-]\\](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ient4lfqx4esikud2h76pywp8ufpb4njg4.png)
![[H+] = (10^(-14))/([OH-])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/iwwfot89qfjhki7bz2ugdpqugh24vqi1v0.png)