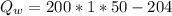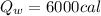Answer:
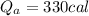
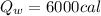
Step-by-step explanation:
From the question we are told that
Mass of the aluminum container 50 g
Mass of the container and water 250 g
Mass of the water 200 g
Initial temperature of the container and water 20°C
Temperature of the steam 100°C
Final temperature of the container, water, and condensed steam 50°C
Mass of the container, water, and condensed steam 261 g
Mass of the steam 11 g Specific heat of aluminum 0.22 cal/g°C
a) Heat energy on container
Generally the formula for mathematically solving heat gain
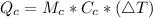
Therefore imputing variables we have
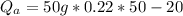
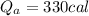
b) Heat energy on water
Generally the formula for mathematically solving heat gain
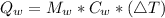
Therefore imputing variables we have