Use Charle's Law because we are dealing with volume and temperature.
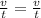
v₁= 3017.0 ml
t₂ = 0
v₂= ???
t₂= 273°C
Change all mL's to L because the question says "What is the volume (L)...".
3017.0 mL = 3.017 L
Change the temperature into Kelvins: Kelvin = Celsius + 273.15
0°C = 273.15 K
273°C = 546.15 K
Now you can plug it in the formula:
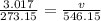
Then, do cross multiplication.
(3.017) (546.15) = (273.15) v₂
1,647.73 = (273.15 K) v₂
Divide 273.15 on both sides
6.0296 L= V₂