Answer : The correct option is, (B) multiplying the equation first by 2.
Explanation :
The balanced final chemical reaction will be,
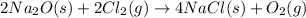
The two intermediate chemical reaction are give :
(1)
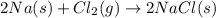
(2)
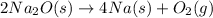
First multiplying the equation (1) by 2, we get
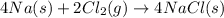
Now adding both the equations, we get
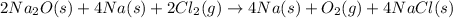
The final chemical reaction are :
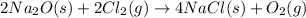
Hence, the correct option is, (B) multiplying the equation first by 2.