Answer : The correct option is, (1)
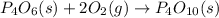
Explanation :
The two intermediate chemical equations are :
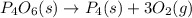 ..............(1)
..............(1)
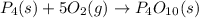 .............(2)
.............(2)
Now adding equation (1) and equation (2), we get
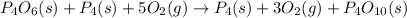
Now we will cancel the
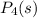 from both the sides of the equation, we get
from both the sides of the equation, we get
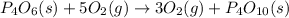
Now we will subtracting
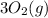 from the
from the
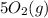 , we get
, we get
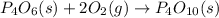
This is the final chemical equation.