Answer : The concentration of
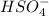 ,
,
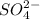 and
and
 are 0.29 M, 0.061 M and 0.061 M respectively.
are 0.29 M, 0.061 M and 0.061 M respectively.
Explanation :
First we have to calculate the concentration of
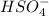
The dissociation of
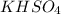 is:
is:
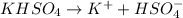
As, 1 mole of
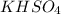 gives 1 mole of
gives 1 mole of
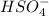
So, 0.35 M of
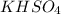 gives 0.35 M of
gives 0.35 M of
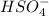
Now we have to determine the concentration of
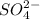 and
and
 .
.
The dissociation of
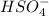 is:
is:
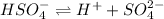
Initial conc. 0.35 0 0
At eqm. (0.35-x) x x
The expression of acid dissociation constant will be:
![K_a=([H^+][SO_4^(2-)])/([HSO_4^-])](https://img.qammunity.org/2020/formulas/chemistry/high-school/qj7008rsyo24vc8sek0ijwu0jd5dgukhfr.png)
Now put all the given values in this expression, we get:
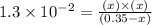
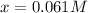
Thus, the concentration of
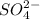 = x = 0.061 M
= x = 0.061 M
The concentration of
 = x = 0.061 M
= x = 0.061 M
The concentration of
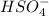 = 0.35 - x = 0.35 - 0.061 = 0.29 M
= 0.35 - x = 0.35 - 0.061 = 0.29 M