Answer : The order of temperature change observed from least change to most change is:
Water < Aluminum < Copper < Brass < Silver < Platinum
Explanation :
Specific heat capacity : It is defined as the amount of heat absorbed by one gram of a substance to raise its temperature by one degree Celsius.
Formula used for specific heat capacity :
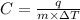
where,
C = specific heat capacity
m = mass of a substance
q = heat required
 = change in temperature of substance
= change in temperature of substance
As per question, each material is heated for the same period of time that means amount of heat is same and mass of substance is also same. So, the change in temperature depends only on specific heat.
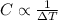
As, there is a inverse relation between the specific heat and temperature.
As, the more is the specific heat of substance, the less will be the temperature change and vice-versa.
The order of temperature change observed from least change to most change is:
Water < Aluminum < Copper < Brass < Silver < Platinum