6.52 × 10⁴ L. (3 sig. fig.)
Step-by-step explanation
Helium is a noble gas. The interaction between two helium molecules is rather weak, which makes the gas rather "ideal."
Consider the ideal gas law:
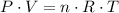 ,
,
where
 is the pressure of the gas,
is the pressure of the gas,
 is the volume of the gas,
is the volume of the gas,
 is the number of gas particles in the gas,
is the number of gas particles in the gas,
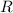 is the ideal gas constant, and
is the ideal gas constant, and
 is the absolute temperature of the gas in degrees Kelvins.
is the absolute temperature of the gas in degrees Kelvins.
The question is asking for the final volume
 of the gas. Rearrange the ideal gas equation for volume:
of the gas. Rearrange the ideal gas equation for volume:
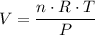 .
.
Both the temperature of the gas,
 , and the pressure on the gas changed in this process. To find the new volume of the gas, change one variable at a time.
, and the pressure on the gas changed in this process. To find the new volume of the gas, change one variable at a time.
Start with the absolute temperature of the gas:
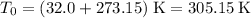 ,
,
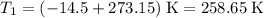 .
.
The volume of the gas is proportional to its temperature if both
 and
and
 stay constant.
stay constant.
 won't change unless the balloon leaks, and
won't change unless the balloon leaks, and- consider
 to be constant, for calculations that include
to be constant, for calculations that include
 .
.
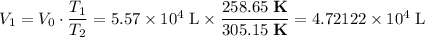 .
.
Now, keep the temperature at
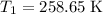 and change the pressure on the gas:
and change the pressure on the gas:
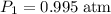 ,
,
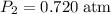 .
.
The volume of the gas is proportional to the reciprocal of its absolute temperature
 if both
if both
 and
and
 stays constant. In other words,
stays constant. In other words,
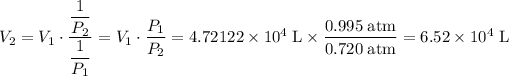
(3 sig. fig. as in the question.).
See if you get the same result if you hold
 constant, change
constant, change
 , and then move on to change
, and then move on to change
 .
.