Hello!
The answer is: the element have 51 neutrons.
Why?
The atomic mass number of an element is equal to the sum of the number of protons and neutrons, also we need to remember that the atomic number is equal to the proton number.
So, to calculate how many neutrons does the element have, we need to write the following equation:
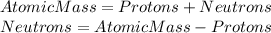
Where:
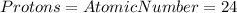
Hence,
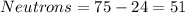
So, the element have 51 neutrons.
Have a nice day!