Answer: Option (D) is the correct answer.
Step-by-step explanation:
The distribution of electrons of an element in their atomic orbitals is known as electronic configuration.
For example, atomic number of neon is 10 and its electronic configuration is
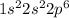 .
.
According to Aufbau's principle, in ground state atomic orbital of an atom starts to fill from the lowest energy level.
So, when highest energy level of an atom is completely filled then it becomes unreactive in nature as then it neither needs to gain or lose electrons.
Hence, we can conclude that a filled highest occupied principal energy level electron configurations is most likely to result in an element that is relatively inactive.