Hello!
The answer is: There are 3.42 moles of MgCl2 in 326g of the compound.
Why?
Assuming that the compound is MgCl2, we can find how many moles of the compound are in 326 g of the same compound, calculating the molar mass of the compound, so:
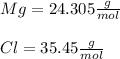
Then,
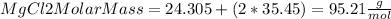
Therefore, to calculate how many moles are in 326 of the compound, we can use the following equation
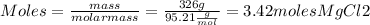
Hence,
There are 3.42 moles of MgCl2 in 326g of the compound.
Have a nice day!