Answer : The correct option is, I and II
Explanation :
According to the Arrhenius theory, an acid is a substance that ionizes in the water to give hydronium ion
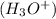 or hydrogen ion
or hydrogen ion
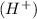 and a bases is a substance that ionizes in the water to give hydroxide ion
and a bases is a substance that ionizes in the water to give hydroxide ion
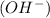 .
.
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
According to the Lewis acid-base theory, an acid is a sustenance that accepts pairs of electrons and the a base is a substance that donates pairs of electrons.
As per question, the Arrhenius and Bronsted Lowry are the definitions of acids and bases that focuses on the transfer of the
 hydrogen particles.
hydrogen particles.
Hence, the correct option is, I and II