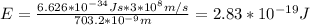Answer:
Energy = 2.83*10^-19 J
Step-by-step explanation:
Given:
Wavelength (λ) emitted by a Neon atom = 703.2 nm
To determine:
Energy (E) of the photon of light
Step-by-step explanation:
Energy (E) pf a photon is related to its wavelength (λ) by the Planck's equation:
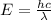
where h = Planck's constant = 6.626*10^-34 Js
c = speed of light = 3*10^8 m/s