Answer:
You will need 15.27 mL of the stock solution ( concentrated solution)
Step-by-step explanation:
Dilution is the procedure used to prepare a less concentrated solution from a more concentrated solution. It consists of adding solvent to an existing solution. Then the amount of solute does not vary, but the volume of the solvent does: when more solvent is added, the concentration of the solute decreases, as the volume of the solution increases.
There is a simple formula to calculate exactly how much stock solution and water are needed to prepare a given dilution:
Vi*Ci=Vf*Cf
The subscript i indicates initial dissolution, which is the concentrated solution, and the subscript f means final dissolution, which is the least concentrated or diluted.
Then:
- Vi = Volume of the mother solution = Volume of the necessary mother solution.
- Ci = Concentration of the stock solution.
- Vf = Volume to be prepared from the final or diluted solution.
- Cf = Concentration of the final or diluted solution.
So, in this case:
- Vi = ?
- Ci = 4.5x10⁻⁴ M
- Vf = 275 mL
- Cf = 2.50x10⁻⁵ M
Replacing you get:
Vi*4.5x10⁻⁴ M= 275 mL*2.50x10⁻⁵ M
Resolving:
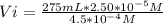
Vi= 15.27 mL
You will need 15.27 mL of the stock solution ( concentrated solution)