Answer : The pH after the addition of 28.0 ml of
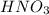 is, 8.1
is, 8.1
Explanation :
First we have to calculate the moles of
 and
and
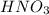 .
.
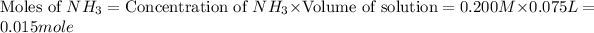
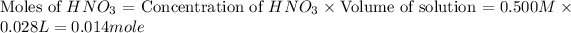
The balanced chemical reaction is,
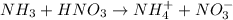
Moles of
 left = Initial moles of
left = Initial moles of
 - Moles of
- Moles of
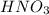 added
added
Moles of
 left = 0.015 - 0.014 = 0.001 mole
left = 0.015 - 0.014 = 0.001 mole
Moles of
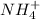 = 0.014 mole
= 0.014 mole
Now we have to calculate the
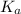 .
.
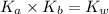
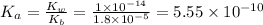
Now we have to calculate the

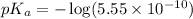
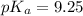
Now we have to calculate the pH by using Henderson-Hasselbalch equation.
![pH=pK_a+\log ([NH_3])/([NH_4^+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/gw9o0o6ovdm1q8fwy37gmsqtyak0dhy413.png)
Now put all the given values in this expression, we get:
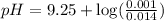
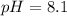
Therefore, the pH after the addition of 28.0 ml of
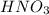 is, 8.1
is, 8.1