Answer:
sample B is the one with the highest kinetic energy, so it should have a higher temperature
Step-by-step explanation:
The temperature of a body is related to the internal energy of a body, if the body is a gas, this internal energy is directly related to the temperature, so for the gases the energy inside a body and the temperature are directly related
An expression for this gas relation is that thermal energy equals kinetic energy
ET = K
3
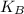 T = ½ m v²
T = ½ m v²
T = K/3
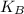
Where ET in thermal energy and
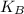 is Boltzmann's constant, in 3 it is because it is a three-dimensional system
is Boltzmann's constant, in 3 it is because it is a three-dimensional system
Therefore, the body with the highest kinetic energy must have a higher temperature
When we observe your answers, sample B is the one with the highest kinetic energy, so it should have a higher temperature