Concentration "molarity" of H₂SO₄ in this solution:
5 × 10⁻³ mol / dm³.
Step-by-step explanation
What's the concentration of H⁺ ions in this solution?
![[\text{H}^(+)] = 10^{-\text{pH}}](https://img.qammunity.org/2020/formulas/chemistry/high-school/cbbmqr0rzgykmesnwgxt5ogmz200ya866k.png) ,
,
where
![[\text{H}^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/go1cuj0of90izmo1vzs8if0lg4zcse0acu.png) is in the unit mol / dm³.
is in the unit mol / dm³.
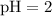
![[\text{H}^(+)] = 10^(-2) \;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/afuurep4o3je81qvn8c1ats1tppfn7aoi1.png) .
.
What's the concentration "molarity" of H₂SO₄ in this solution?
Sulfuric acid H₂SO₄ is a strong acid. Note the subscript "2". Each mole of this acid dissolves in water to produce two moles of H⁺ ions. It takes only
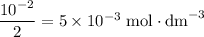 of H₂SO₄ to produce twice as much H⁺ ions.
of H₂SO₄ to produce twice as much H⁺ ions.
As a result, the molarity of H₂SO₄ is 5 × 10⁻³ mol / dm³ or 0.005 M.