Answer:
V = 338.8
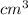
Explanation:
Basically manipulate the ideal gas law.
PV = nRT
We know n and R are constant so we can cross that out. We get:
PV = T or
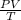
Let's plug in the values to get:
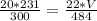
We simplify them to get:
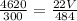
We cross multiply:
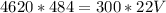
Simplify:
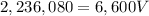
Solve for V by dividing both sides by 6,600.
Hope it helps! (: