0.040 mol / dm³. (2 sig. fig.)
Step-by-step explanation
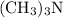 in this question acts as a weak base. As seen in the equation in the question,
in this question acts as a weak base. As seen in the equation in the question,
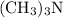 produces
produces
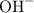 rather than
rather than
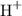 when it dissolves in water. The concentration of
when it dissolves in water. The concentration of
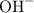 will likely be more useful than that of
will likely be more useful than that of
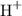 for the calculations here.
for the calculations here.
Finding the value of
![[\text{OH}^(-)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/sjaqu1y8fibz6q9bhe4ft7tvpj2ibpzcpc.png) from pH:
from pH:
Assume that
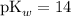 ,
,
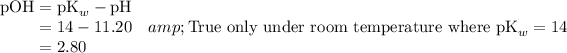 .
.
![[\text{OH}^(-)] =10^{-\text{pOH}} =10^(-2.80) = 1.59\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/pb3cki52h9b8cijqkav7hopjk5rs1qpwc7.png) .
.
Solve for
![[(\text{CH}_3)_3\text{N}]_\text{initial}](https://img.qammunity.org/2020/formulas/chemistry/high-school/ypx30coo645midxfhh9jszi47d31wpy8w0.png) :
:
![\frac{[\text{OH}^(-)]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^(+)]_\text{equilibrium}}{[(\text{CH}_3)_3\text{N}]_\text{equilibrium}} = \text{K}_b = 1.58* 10^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/50l96fubrefj2owk4kwmbj7mwywvxny3ay.png)
Note that water isn't part of this expression.
The value of Kb is quite small. The change in
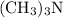 is nearly negligible once it dissolves. In other words,
is nearly negligible once it dissolves. In other words,
![[(\text{CH}_3)_3\text{N}]_\text{initial} = [(\text{CH}_3)_3\text{N}]_\text{final}](https://img.qammunity.org/2020/formulas/chemistry/high-school/oqajluwxcdj47vq78022fp5jp3nqvltggl.png) .
.
Also, for each mole of
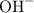 produced, one mole of
produced, one mole of
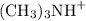 was also produced. The solution started with a small amount of either species. As a result,
was also produced. The solution started with a small amount of either species. As a result,
![[(\text{CH}_3)_3\text{NH}^(+)] = [\text{OH}^(-)] = 10^(-2.80) = 1.58* 10^(-3)\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/jbbbrcplj9zcch4c621by14q9dyxsuf796.png) .
.
![\frac{[\text{OH}^(-)]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^(+)]_\text{equilibrium}}{[(\text{CH}_3)_3\text{N}]_\textbf{initial}} = \text{K}_b = 1.58* 10^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/lxbbfksj9yajlqpydc13h89bt29duzny5o.png) ,
,
![[(\text{CH}_3)_3\text{N}]_\textbf{initial} =\frac{[\text{OH}^(-)]_\text{equilibrium}\cdot[(\text{CH}_3)_3\text{NH}^(+)]_\text{equilibrium}}{\text{K}_b}](https://img.qammunity.org/2020/formulas/chemistry/high-school/wu0466xlo8rq35fzcm9xeo4zxcikmeu3uz.png) ,
,
![[(\text{CH}_3)_3\text{N}]_\text{initial} =((1.58*10^(-3))^(2))/(6.3*10^(-5)) = 0.040\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/iiscgad6yl6a5lpt3vroudyawnk1bvsis2.png) .
.