1.
Answer:
Metallic plates emit electrons only when light of a certain minimum frequency shines on them.
Step-by-step explanation:
As we know by Einstein's equation of energy
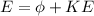
here we know that
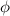 = work function of metal
= work function of metal
KE = kinetic energy of ejected electrons
E = energy of incident light
So here electrons will eject only when light of sufficient minimum energy will incident on the metal plate.
2.
Answer:
showed that individual photons have wave characteristics
Step-by-step explanation:
Taylor did experiment with the source of light with very small intensity of light and then got the result of interference of light.
So here his experiment concluded that all light will show interference only due to one photon also.
So his experiment shows that one photon will give wave characteristics