Answer: Group 2, because it has two electrons in the 3s orbital.
Step-by-step explanation:
Electronic configuration represents the total number of electrons that a neutral element contains.Thus for element with atomic number 12 contains 12 electrons.
The electrons are filled according to Afbau's rule in order of increasing energies and thus the electronic configuration is :
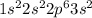
As the element belongs to s-block, as last electron enters s orbital, its group number = Number of valence electrons (or outermost shell electrons), which is 2.
Thus group number is 2.