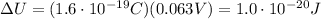Answer:
Step-by-step explanation:
The change in the ion's electric potential charge as it moves from inside to outside the cell is:
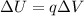
where
q is the charge of the ion
 is the potential difference
is the potential difference
- The charge of the ion is +e, since it is positively charged, so
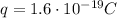
- The potential difference is
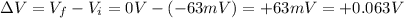
So, the change in the ion's electric potential energy is