Answer:
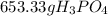
Step-by-step explanation:
Hello,
In this case, by considering the given chemical reaction and the molar mass for phosphoric acid and the 4 to 6 relationship between phosphoric acid and water respectively, the required grams are result:
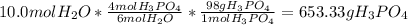
Best regards.