The equation is missing. So all the options can be possible.
Step-by-step explanation:
CO is termed as carbon monoxide. Mostly CO is formed by the absence of desired amounts of oxygen on reaction with carbon based molecules. So CO will be formed as the by products instead of forming
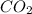 as byproducts due to less oxygen presence.
as byproducts due to less oxygen presence.
So CO can form as product. Even CO can be available naturally during volcanoes burning which releases
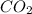 on reaction with atmosphere. Thus in this case, CO will act as reactant. So as CO can be obtained in a product and also a reactant to form
on reaction with atmosphere. Thus in this case, CO will act as reactant. So as CO can be obtained in a product and also a reactant to form
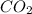 . It can act as both a reactant and a product.
. It can act as both a reactant and a product.
In order to understand the status of CO in a chemical reaction equation, the position of CO in the chemical equation should be noted. It is known that the left side of chemical reaction equations contains the reactants for the reaction and the right side of the chemical reaction equation denotes the products formed from the reactants.
So if CO is placed in the left side, then it is a reactant and if it is placed in right side, it will product and in some cases, if CO is placed in both the sides then CO is acting as a catalyst i.e., reactant and product in that reaction.