8,486 grams. (4 sig. fig.) That's 8.486 × 10³ grams using scientific notations.
Step-by-step explanation
Relative atomic mass from a modern periodic table:
Sodium is a group 1 alkali metal. It forms positive ions of charge +1.
Sulfide ion is an ion made from a single sulfur atom. Sulfur is in group 16 and is 18 - 16 = 2 electrons short of an octet. An sulfur atom would gain two electrons when it forms a sulfide ion. The charge on that ion will be -2.
Charges on the two ions balance in sodium sulfide, which is overall neutral. Two
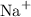 ions will pair up with one
ions will pair up with one
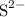 ion. This 2:1 ratio gives the formula:
ion. This 2:1 ratio gives the formula:
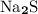 .
.
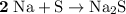 .
.
It takes two sodium atoms to form one formula unit of sodium sulfide
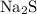 .
.
How many moles of sodium atoms in 5,000 grams of sodium metal?
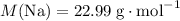 .
.
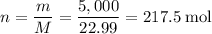 .
.
How many moles of sodium sulfide formula units will that 217.5 moles of Na atoms make?
In case sulfur is in excess, every two moles of Na atoms will make one formula unit of sodium sulfide. 217.5 moles of Na atoms will make 108.8 moles of sodium sulfide.
What's the mass of 108.8 moles of sodium sulfide?
Molar mass of sodium sulfide:
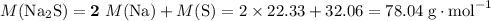 .
.
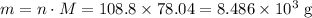 .
.