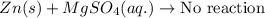Answer: The correct answer is no reaction will occur.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive metal displaces a less reactive metal from its chemical reaction.
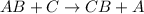
C is more reactive element than element A.
The reactivity of metals is judged by the series known as reactivity series. Elements lying above in the series are more reactive than the elements lying below in the series.
We are given:
Magnesium lies above zinc in the reactivity series. So, if zinc particles lie in the solution of magnesium sulfate. The zinc particles will not displace magnesium ions from its solution because zinc is less reactive than magnesium.
Hence, no reaction will occur between them.