Answer : The correct option is, (A)
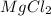
Explanation :
Binary ionic compound : It is defined as the compound that is made up of the ions of the two different elements in which one element is a metal and another element is a non-metal.
From the given options, option (A)
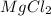 is a binary ionic compound because in this compound Mg is a metal which combine with the a non-metal element that is chlorine.
is a binary ionic compound because in this compound Mg is a metal which combine with the a non-metal element that is chlorine.
While the other options are the covalent compounds that are formed by the combination of non-metal and non-metal elements.
Hence, the
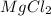 is a binary ionic compound.
is a binary ionic compound.